Rank the Double Bonds Below in Terms of Increasing Stability
Rank the conformers of butane in order of decreasing stability putting the most stable first. Rank the following alkenes in order of increasing stability of the double bond towards addition of HBr.

Oneclass Rank The Double Bonds Below In Terms Of Increasing Stability
The order of decrease of the stability of the carbocations is.

. 2 1 3 C. Rank the double bonds below in terms of increasing stability OneClass. Two σ bonds and one π bond form.
Rank the radicals shown in order of decreasing stability with the most stable radical at the top. Carbon of the double bond and this is the least stable. Rank each of the following C-C sigma bond combinations in order of increasing bond length beginning with the shortest at the top of the list.
Explore the factors that affect the stability of alkenes and examples of. 4 pts -B NH2 А e. I II III CH 3 CH 3 CH 3 CH 3 CH 2 CH 3 55 Organic chemistry is about the interaction between ELECTRON-RICH atoms and molecules NUCLEOPHILES and ELECTRON-DEFICIENT atoms or molecules ELECTROPHILES.
2 3 1 6. 4 pts -B NH2 А e. Solution for Rank the following carbocations in order of increasing stability.
A 2 B 3 C 4 D 5 E 6. The double bonds and triple bonds present between any 2 car. The thermodynamic product is less stable.
GET 20 OFF GRADE YEARLY SUBSCRIPTION. A B D There is a positive charge in D adjacent to a double bond. The reason being is the positive charge directly on a double bond so we cant draw any the resonant structures.
Bond 2 Bond 3. Step 1 of 5. If the two atoms attached to the double bond carbon are identical designated A and B below look at all the atoms directly attached to the identical atoms in questions designated A-1.
Rank each of the following alkyl halides in order of increasing rate of S. Solutions for problems in chapter 7 1P. When youre comparing three carbon hydrogen single bonds as we are in part B we need to look at the hybridization sze of the carbons involved in these bonds and remember other way that as p character works So uh on SP carbon is gonna be 50 s character So.
Rank the double bonds below in terms of increasing stabilitya b c. Start your trial now. But carbocation 5 is vinylic carbocation positively charged carbon is sp 2 hybridized ie.
The increasing bond length order. Given that arachidonic acid is an acyclic carboxylic acid that contains no carbon-carbon triple bonds how many carbon-carbon double bonds are present. Rank the radical classes below in order of increasing stability with the least stable at the top.
Assign configurations EIZ to the double bonds labeled A and B below. A carbon atom with an unpaired electron is directly adjacent to an Correct Answer double bond. The stability of the alkenes increases as the number of R groups bonded to the double bond carbons increases.
If the high priority atoms are on opposite sides. A The increasing order of bond strength is. Allylic carbocations are able to share their burden of charge with a nearby group through resonance.
First week only 499. Priority of common atoms. Two π bonds break.
Assuming the rate of reaction in terms of that one reactant is given by Rate K A. Hence the order of increasing stability. On each double bond to which.
One σ bond and one π bond form. Y 1 3. Rank the following alkenes in terms of increasing stability 1 least.
View a21jpg from CHEM 123 at University of Southern Mindanao. Triple is gonna be the shortest a double in the middle and single bond the longest. Up to 256 cash back Get the detailed answer.
Solution for Rank the following bonds in order of increasing bond length. Double bond it is designated as E entgegen across. Solution for N-Weve got the study and writing resources you need for your assignments.
54 PROBLEM 7-48a Rank the double bonds below in order of increasing stability. I Br Cl S F O N C H If both high priority atoms are on the same side of the double bond it is designated Z. Three π bonds break.
Acidity bond strength boiling point solubility heat of combustion conformation constitution and reactivity. 1 2 3 H H. Bond 1 Bond 3 Bond 2.
A simple allylic system will have just one pi bond Though you may see multiple resonating pi bonds. Trans alkene is more stable than the cis alkene because the groups bonded to the double bond carbons are farther apart reducing the steric interactions. 1Look at the atoms directly attached to each carbon of the double bond.
Three π bonds break. A vinyl carbocation has a positive charge ON THE SAME carbon as the double bond. F 3 1 2 C.
The prostaglandin precursor arachidonic acid has the molecular formula C20H32O2. Step 1 of 3. 1 2 3 b.
Rank the molecules in each set below according to the trends observed for the physical and chemical properties indicated. - sp3-sp2 sigma bond of propene - sp-sp sigma bond of 13-butadiyne - sp2-sp2 sigma bond of 13-butadiene - sp3-sp3 sigma bond of ethane. Since we know that if the of S character increases then the bond strength gets increased and the bond length gets decreased.
Which of the following statements about the mechanism of the Diels-Alder reaction is true. 4 most stable. Rank the double bonds below in terms of increasing stability LIMITED TIME OFFER.
This is VERY VERY unstable and ranks under a methyl carbocation in stability. Organic Chemistry 9th Edition Edit edition Solutions for Chapter 7 Problem 48E. This results in resonance stabilization of the molecule making it more stable than A and B.
Rank the double bonds below in terms of increasing stability a. One σ bond and two π bonds form. Alkenes are hydrocarbon chains using at least one double bond per chain.
Between A and B A is a primary carbocation and B is a secondary carbocation and tertiary carbocations secondary primary carbocations in terms of stability. Rank them according to decreasing atomic number. 4 3 2 1 5.
I III II Rank the following alkanes in order of decreasing boiling point putting the alkane with the highest boiling point first.
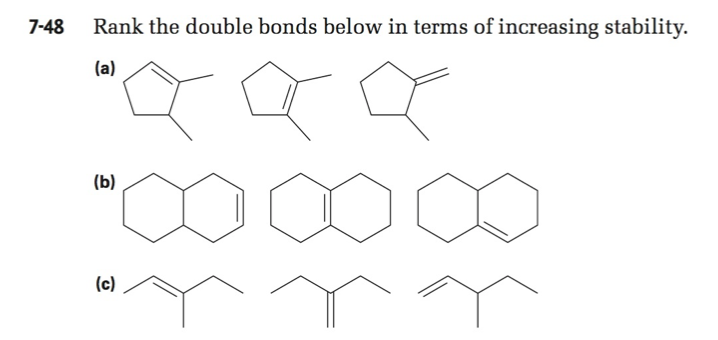
Solved 7 48 Rank The Double Bonds Below In Terms Of Chegg Com

Oneclass Rank The Double Bonds Below In Terms Of Increasing Stability
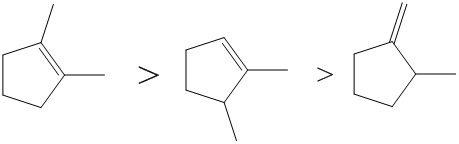
Solved Rank The Double Bonds Below In Terms Of Increasing Stabili Chegg Com
No comments for "Rank the Double Bonds Below in Terms of Increasing Stability"
Post a Comment